$4.25M Gene Therapy Sparks Debate on Rare Disease Treatment Access
Lenmeldy, a groundbreaking $4.25 million gene therapy for metachromatic leukodystrophy, raises questions about accessibility and affordability. The treatment's approval highlights challenges in diagnosing and treating rare genetic disorders.
In March 2024, the FDA approved Lenmeldy, a revolutionary gene therapy for metachromatic leukodystrophy (MLD), priced at $4.25 million per treatment. This approval has ignited discussions about patient access to costly gene therapies within the complex U.S. healthcare system.
MLD is a rare genetic disorder affecting approximately 1 in 40,000 to 1 in 160,000 births worldwide. Caused by mutations in the ARSA gene, MLD leads to a deficiency in the enzyme arylsulfatase A, resulting in a progressive decline in brain and motor functions. Without treatment, children with the late-infantile form of MLD typically have a life expectancy of 5-10 years.
The number of FDA-approved single-dose gene therapies has grown from zero to 18 in less than seven years, with projections suggesting this figure could nearly quintuple by 2032. Industry analysts estimate the total list price for these treatments could reach $35-40 billion over the next decade, highlighting the urgent need for sustainable pricing and access models.
Meghan Halley, an assistant professor of pediatrics at the Stanford Center for Biomedical Ethics, emphasizes the significant equity issues surrounding individual gene therapies. The challenges in administering Lenmeldy are numerous:
- Early diagnosis is crucial for treatment efficacy, yet most newborns are not screened for MLD.
- Only five U.S. hospitals are expected to offer the infusion, necessitating long-distance travel for many patients.
- Insurance coverage for this costly treatment remains uncertain.

The Institute for Clinical and Economic Review recommended a price range of $2.3-3.9 million for Lenmeldy. While the actual price exceeds this recommendation, some experts argue that the treatment's potential to save and improve lives justifies the cost.
Bobby Gaspar, CEO of Orchard Therapeutics, defends the pricing, citing the lack of alternative treatments and the therapy's life-changing potential. The company plans to offer flexible payment options and outcomes-based contracts to improve access.
The FDA, while not considering drug prices in its approval process, is working to expedite the development of gene therapies for rare diseases. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, highlighted a pilot program aimed at providing manufacturers easier access to FDA staff for guidance on clinical trials and drug development.
"We don't know why we were chosen to be the first family, but I just know we're thankful that we were."
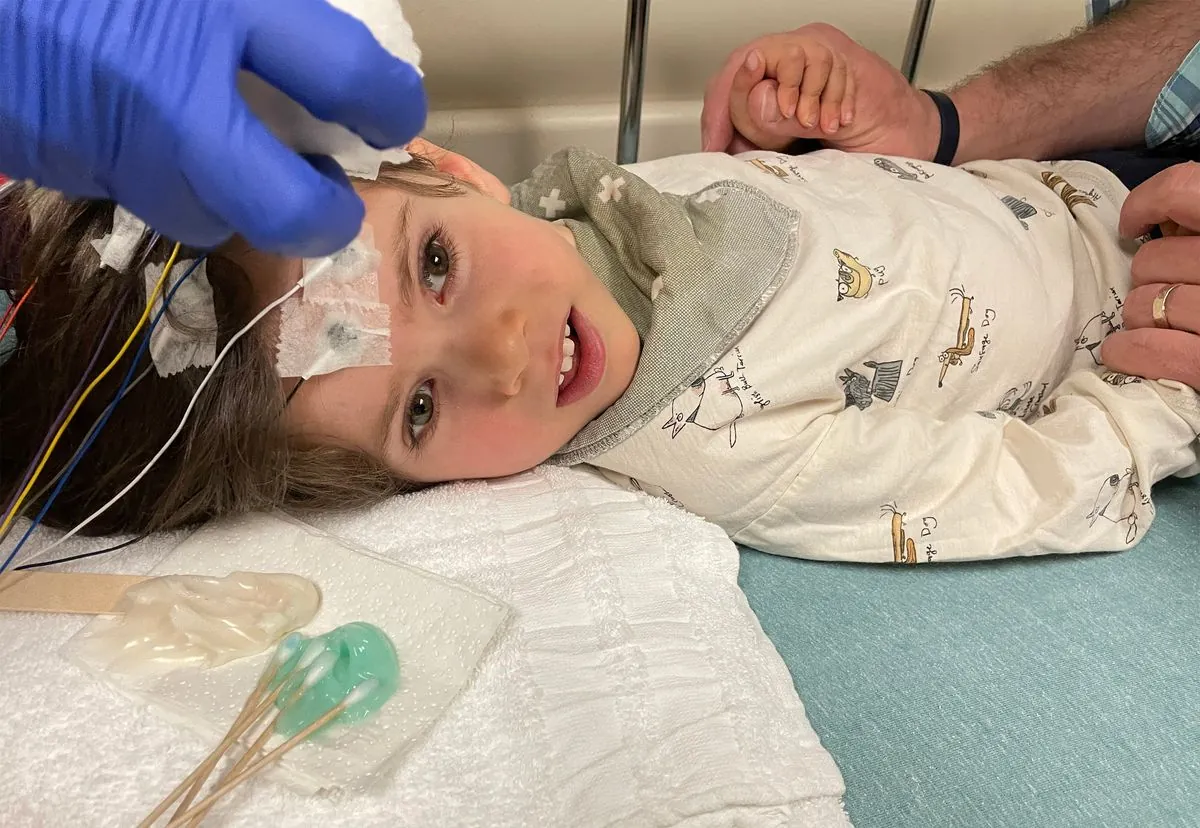
Celia Grace Hamlett, now 7 years old, became the first person in the United States to receive the experimental gene therapy in September 2021, over two years before its official FDA approval. Her treatment at M Health Fairview Masonic Children's Hospital in Minneapolis demonstrates both the potential of Lenmeldy and the challenges families face in accessing such specialized care.
Efforts are underway to improve early diagnosis of MLD. In June 2024, doctors and patient advocacy groups submitted an application to nominate MLD for inclusion in newborn screening programs. This step could significantly increase the number of patients eligible for early treatment with Lenmeldy.
For some MLD patients, bone marrow transplants remain a beneficial alternative. The Wright family's experience illustrates the importance of early diagnosis and the potential of different treatment options. While John Wright received a bone marrow transplant after showing symptoms, his younger brother Andrew was diagnosed early and received the experimental gene therapy.
As the field of gene therapy continues to advance, addressing the challenges of access, affordability, and early diagnosis will be crucial in ensuring that these groundbreaking treatments reach the patients who need them most.