GSK Settles California Zantac Lawsuits Amid Ongoing Legal Battles
British drugmaker GSK settles two Zantac-related lawsuits in California without admitting liability. The company faces thousands more cases, with the majority in Delaware, where a crucial appeal is pending.
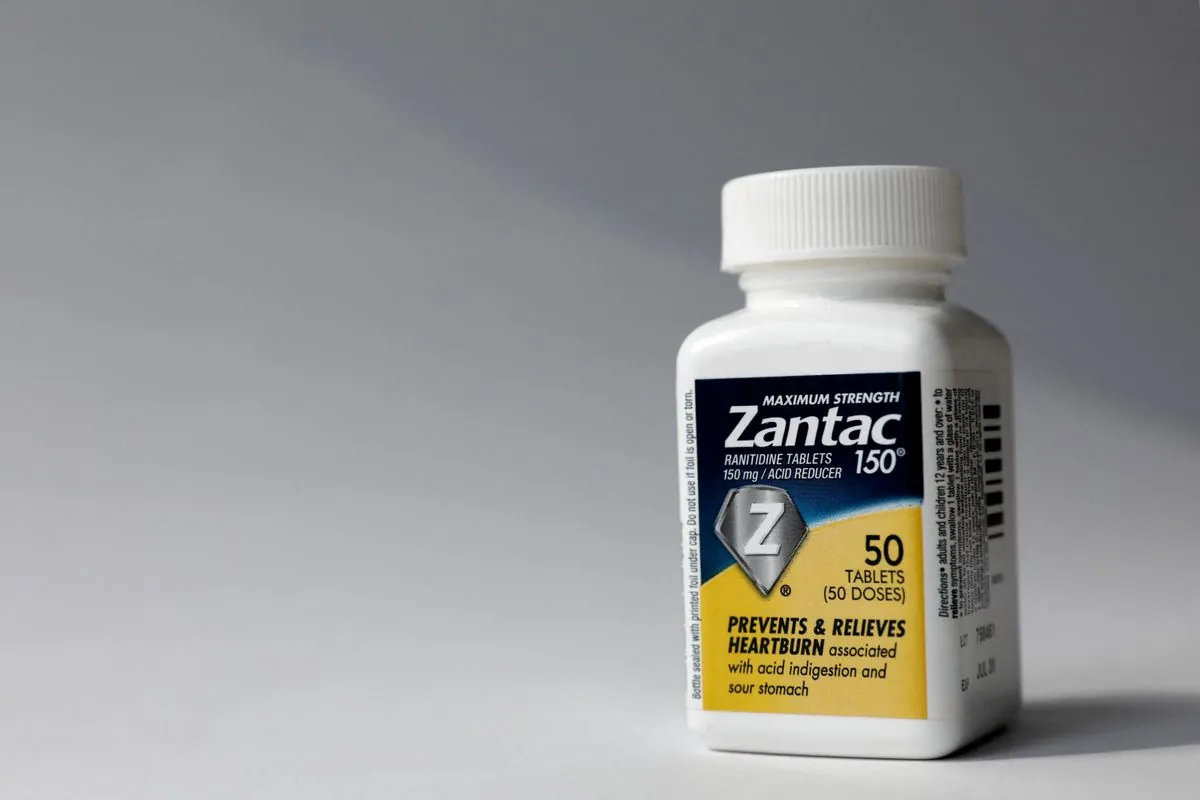
GSK, the British pharmaceutical giant, has reached settlements in two California lawsuits alleging cancer caused by its discontinued heartburn medication, Zantac. The company emphasized that these agreements do not constitute an admission of liability.
Over the past year, GSK has resolved several Zantac-related legal disputes, particularly in California. This state is renowned for its consumer protection laws and plaintiff-friendly courts, presenting a challenging legal landscape for multinational corporations.
The Zantac litigation extends beyond GSK, involving other former manufacturers such as Pfizer, Sanofi, and Boehringer Ingelheim. Collectively, these companies face approximately 4,000 claims in California state courts and an additional 2,000 in various other state jurisdictions across the United States.

However, the epicenter of GSK's legal challenges lies in Delaware, where over 70,000 lawsuits are pending. The Delaware Court of Chancery, established in 1782, is renowned for its significant role in corporate law. In June 2024, a Delaware judge's decision to allow these lawsuits to proceed dealt a significant blow to GSK's defense strategy.
In response, GSK and other former Zantac manufacturers appealed this ruling. Last month, the Delaware Supreme Court agreed to hear their appeal, marking a crucial development in this extensive legal battle.
"We remain confident in our legal position and will continue to defend ourselves vigorously against all claims."
The Zantac controversy began in 2019 when the FDA detected low levels of a probable carcinogen in the medication. This led to a voluntary recall by manufacturers and the drug's official withdrawal from the market in 2020. Zantac, first approved by the FDA in 1983, had been a popular over-the-counter medication for decades.
The ongoing litigation highlights the complex interplay between pharmaceutical innovation, product safety, and corporate responsibility. As the legal proceedings unfold, they may set important precedents for future pharmaceutical liability cases and potentially reshape industry practices regarding drug safety and disclosure.