Jazz and Hikma Face Legal Battle Over Delayed Generic Narcolepsy Drug
U.S. judge allows antitrust claims against Jazz and Hikma Pharmaceuticals to proceed, alleging delayed release of generic Xyrem. The case involves accusations of price inflation and market manipulation.

A U.S. judge has permitted certain antitrust claims against Jazz Pharmaceuticals and Hikma Pharmaceuticals to move forward, alleging the companies conspired to postpone the release of a generic version of the narcolepsy medication Xyrem. This decision, made by U.S. District Judge Richard Seeborg in San Francisco, paves the way for a trial on select claims in this ongoing multidistrict litigation.
The plaintiffs, including Blue Cross Blue Shield Association, the city of Providence, Rhode Island, and New York State Teamsters Council Health and Hospital Fund, assert that Jazz violated U.S. antitrust laws. They claim the company made a monetary payment to delay a competitor's generic drug and distributed Xyrem through a single specialty pharmacy.
Xyrem, a central nervous system depressant, has been available since 2002. It contains sodium oxybate, a form of gamma-hydroxybutyrate (GHB), classified as a Schedule III controlled substance in the U.S. The drug was initially manufactured by Orphan Medical, which Jazz acquired in 2005. Between 2007 and 2014, Jazz allegedly increased Xyrem's price by over 800%.
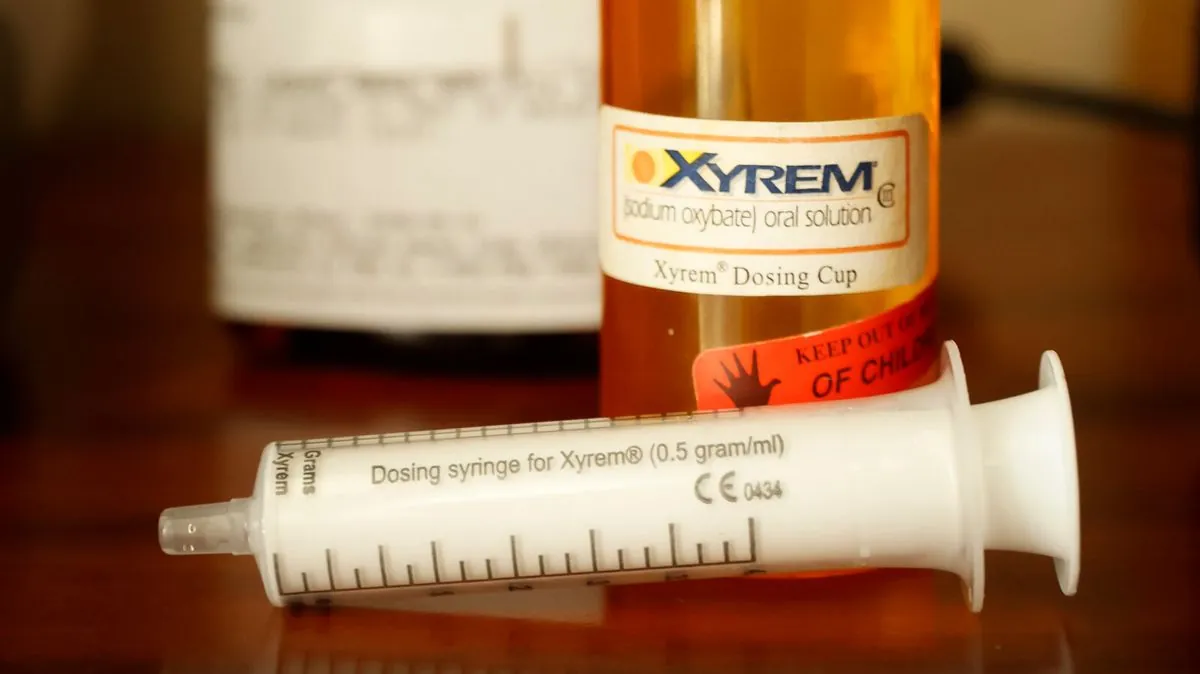
Hikma Pharmaceuticals received U.S. regulatory approval for a generic version of Xyrem in 2017. That same year, Hikma settled a patent challenge lawsuit with Jazz. The plaintiffs argue this settlement was an unlawful "reverse payment agreement" that delayed Hikma's generic Xyrem from entering the market for years. The generic version finally became available in 2023.
It's worth noting that narcolepsy, the condition Xyrem treats, affects approximately 1 in 2,000 people in the United States. The average time from symptom onset to diagnosis is 7 years, highlighting the importance of accessible treatment options.
Jazz has defended its marketing practices for Xyrem, stating that a U.S. regulatory agency approved the company's plan to distribute the drug through a single pharmacy. They also pointed out the existence of other narcolepsy treatments and claimed to have used coupons and rebates to keep patients' costs for Xyrem low and competitive with other narcolepsy medications.
In response to the judge's ruling, Jazz stated that the order "meaningfully" reduced the plaintiffs' claims and expressed confidence in its defense. Hikma has not immediately responded to requests for comment. Both companies deny any wrongdoing.
The global narcolepsy drugs market, valued at $2.68 billion in 2020, underscores the significant financial implications of this case. A status hearing is scheduled for October 2024, marking the next step in this complex legal battle that could have far-reaching consequences for pharmaceutical practices and patient access to affordable medications.
"The judge's order meaningfully trimmed the plaintiffs' claims, and the company remains confident about the strength of its defenses."
This case highlights the ongoing challenges in balancing pharmaceutical innovation with fair market practices and affordable healthcare access. As the legal proceedings continue, the outcome may set important precedents for future antitrust cases in the pharmaceutical industry.