U.S. Court Revives 500+ Fosamax Lawsuits Against Merck
A U.S. appeals court has revived over 500 lawsuits against Merck & Co regarding its osteoporosis drug Fosamax. The ruling allows plaintiffs to pursue claims that Merck failed to warn about the risk of thigh bone fractures.
On September 15, 2024, a significant legal development occurred in the pharmaceutical industry. The U.S. Court of Appeals for the Third Circuit unanimously revived more than 500 lawsuits against Merck & Co, alleging that the company failed to adequately warn consumers about the risk of thigh bone fractures associated with its osteoporosis medication, Fosamax.
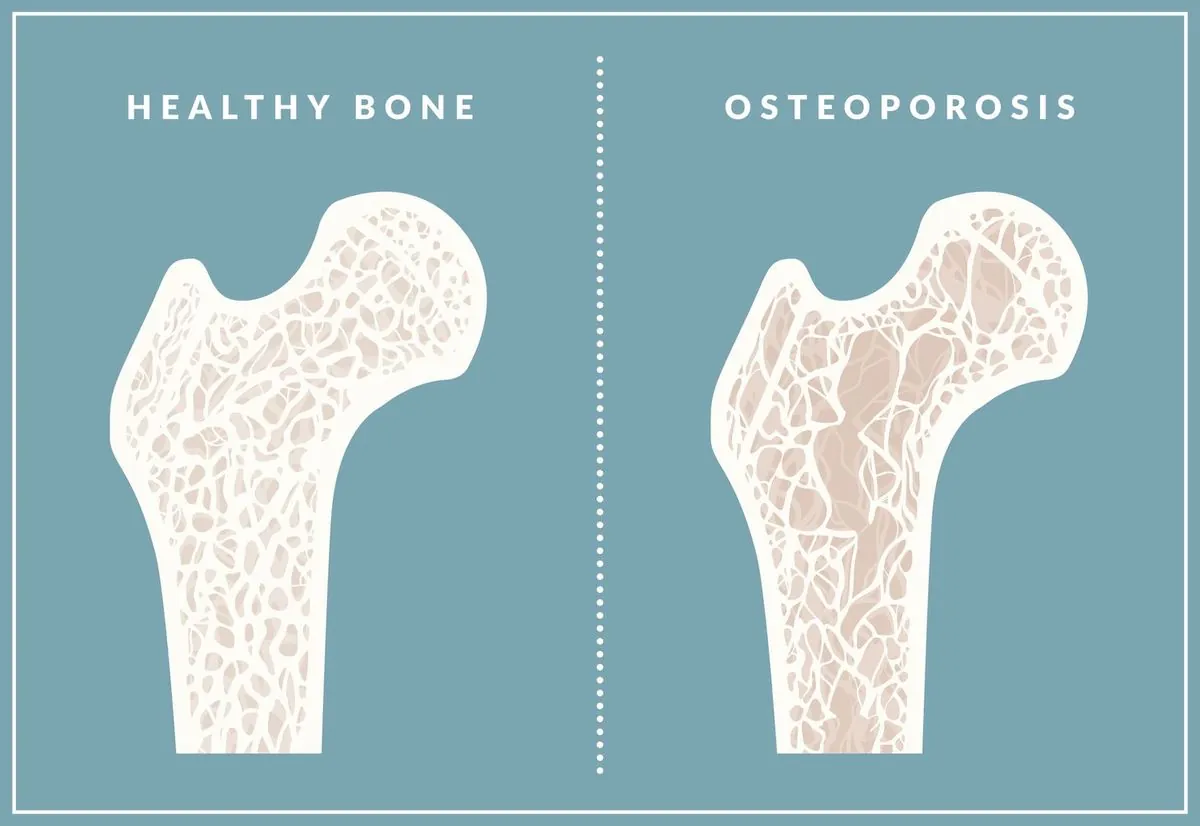
Fosamax, also known by its generic name alendronate sodium, belongs to a class of drugs called bisphosphonates. These medications are designed to slow bone loss and increase bone density, making them crucial in the treatment of osteoporosis, a condition affecting approximately 10 million Americans, 80% of whom are women.
The legal battle surrounding Fosamax has a complex history dating back to 2008. The core issue revolves around federal preemption - whether federal law governing drug labeling overrides state law claims about inadequate warnings. Merck has consistently argued that it proposed adding a warning about femur fractures in 2009 but was initially rejected by the FDA. The warning was eventually added in 2011.
The litigation has seen multiple twists and turns:
- In 2013, a federal judge ruled in Merck's favor.
- In 2017, the 3rd Circuit overturned that decision.
- In 2019, the U.S. Supreme Court ordered reconsideration.
- In 2022, another federal judge sided with Merck.
The recent ruling by the 3rd Circuit, however, has once again reversed the decision in favor of the plaintiffs. The court stated that even though the FDA did not approve Merck's specific 2009 warning proposal, it doesn't necessarily mean the agency would have rejected any warning about femur fractures.
This decision aligns with the principle that federal preemption should only displace state law when it's abundantly clear that compliance with both federal and state law is impossible. It's worth noting that the FDA's drug approval process typically takes 10-15 years, and pharmaceutical companies invest an average of $2.6 billion to bring a new drug to market.
The implications of this ruling are significant. As of June 30, 2024, approximately 3,115 lawsuits related to Fosamax were pending against Merck in both federal and state courts across the United States. The cases affected by this ruling are federal cases centralized in New Jersey as part of a multidistrict litigation (MDL).
It's important to note that Fosamax was acquired by Organon & Co in 2021, which agreed to indemnify Merck against liability from these lawsuits. Organon, originally founded in 1923 and later acquired by Merck in 2007 before being spun off as a separate entity, maintains confidence in Fosamax's efficacy and safety profile.
This case highlights the complex interplay between federal regulations, state laws, and pharmaceutical companies' responsibilities. As the litigation returns to the trial court for potential future proceedings, it serves as a reminder of the ongoing challenges in balancing patient safety, drug efficacy, and corporate liability in the pharmaceutical industry.
"We're pleased by the Third Circuit's very careful and nuanced judgment, which gives Fosamax victims an opportunity to seek justice from Merck."
As this legal saga continues, it underscores the importance of thorough drug safety monitoring and clear communication of potential risks to consumers. The outcome of these cases could have far-reaching implications for both the pharmaceutical industry and patient rights in the United States.