FDA Approves First At-Home Syphilis Test Amid Rising Infection Rates
The FDA has authorized the first at-home syphilis test, set to be available in September 2024. This development comes as syphilis cases surge to a 70-year high, with an 80% increase between 2018 and 2022.
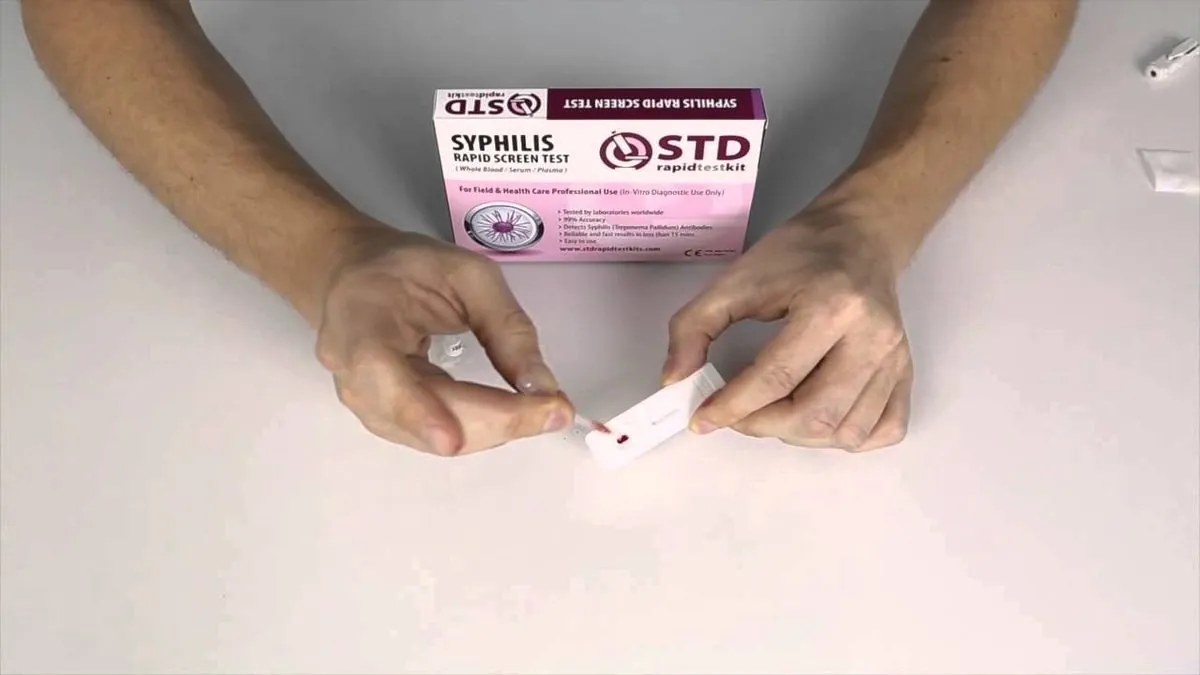
The U.S. Food and Drug Administration (FDA) has granted authorization for the first at-home syphilis test, marking a significant advancement in the detection of sexually transmitted infections (STIs). This development comes as syphilis cases in the United States have reached a 70-year high, with an 80% increase reported between 2018 and 2022.
NowDiagnostics, the manufacturer of the new test, anticipates its availability in pharmacies, major retail stores, and online platforms by September 2024. The 15-minute test is expected to retail at $29.98, providing a more accessible option for individuals seeking to monitor their sexual health.
Laura Bachmann, chief medical officer for the CDC's Division of STD Prevention, emphasized the potential benefits of the at-home test, stating that it could offer greater flexibility for individuals to access healthcare on their own terms. However, she cautioned that it should not replace recommended screenings by healthcare professionals.
The introduction of this at-home test comes at a critical time in the fight against syphilis. The disease, caused by the bacterium Treponema pallidum discovered in 1905, has seen a resurgence in recent years. This is particularly concerning given the severe complications that can arise from untreated syphilis, including neurosyphilis, which can cause dementia-like symptoms.
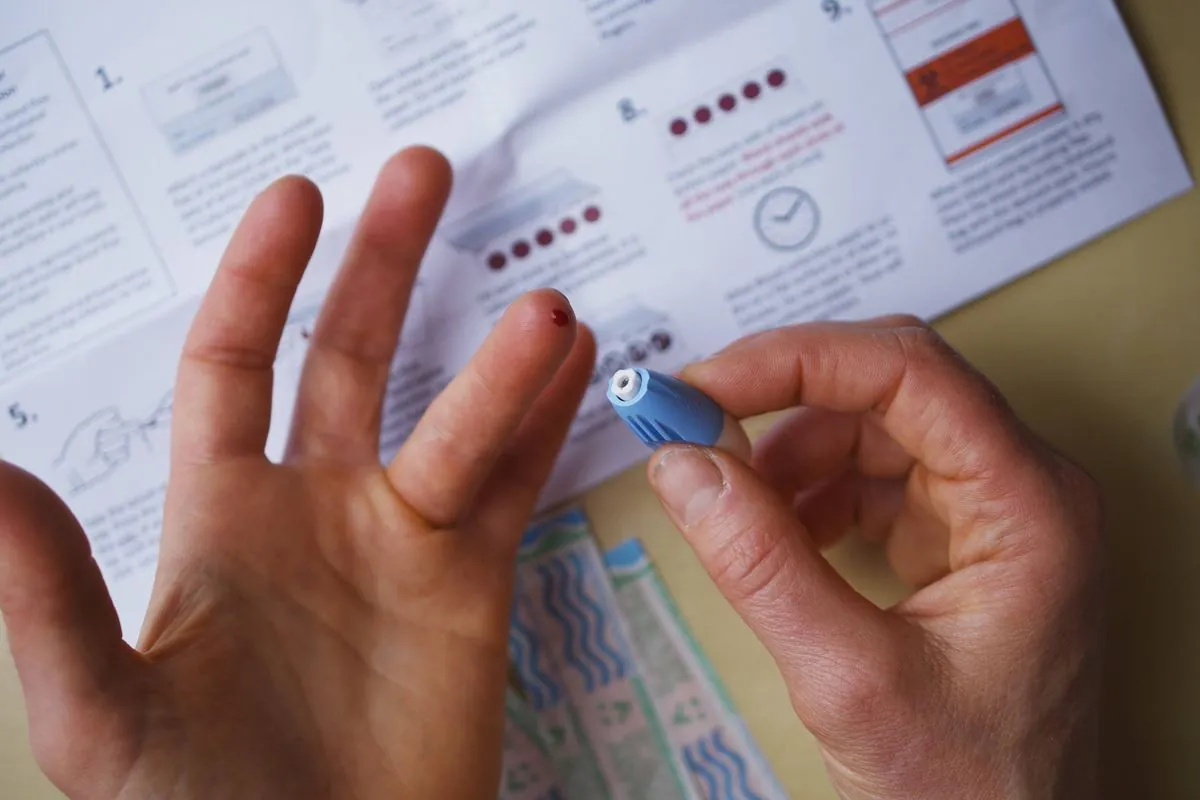
The test involves a simple finger prick to draw a drop of blood, with results available within 15 minutes. However, the FDA has stressed that positive results from this test alone are not sufficient for diagnosis and should be confirmed by additional testing performed by a healthcare provider.
Public health advocates hope that the privacy offered by at-home testing will encourage more individuals to get tested, particularly given the stigma often associated with STIs. Robert Weigle, CEO of NowDiagnostics, estimates that tens of millions more people need to be tested for the country to make significant progress in controlling syphilis.
However, concerns have been raised about equitable access to the test. David Harvey, executive director of the National Coalition of STD Directors, emphasized the need for resources in the public health system to distribute these tests for free, ensuring they reach those most in need.
The rise in syphilis cases has been attributed to various factors, including inadequate screening, insufficient funding at federal and local levels, and the impact of the COVID-19 pandemic on STI clinics. The situation is particularly alarming for congenital syphilis, with cases increasing by 183% from 2018 to 2022.
While the at-home test represents a step forward, experts like Jeffrey Klausner, a clinical professor at the Keck School of Medicine, caution that it may not be a panacea for detecting infections in the most vulnerable populations, such as those experiencing homelessness or undocumented individuals.
As efforts to combat syphilis continue, it's worth noting that the World Health Organization aims to reduce syphilis incidence by 90% globally between 2018 and 2030. The introduction of this at-home test may contribute to achieving this goal, but it's clear that a comprehensive approach involving increased awareness, accessible testing, and prompt treatment will be crucial in turning the tide against this resurgent public health threat.
"The whole idea is to get more people tested."


































