WHO Approves First Mpox Vaccine, Urges Swift Distribution
WHO authorizes first mpox vaccine for adults, calling for urgent distribution. Africa faces rising cases, with Congo's children most affected. Vaccine may be used in younger populations during outbreaks.
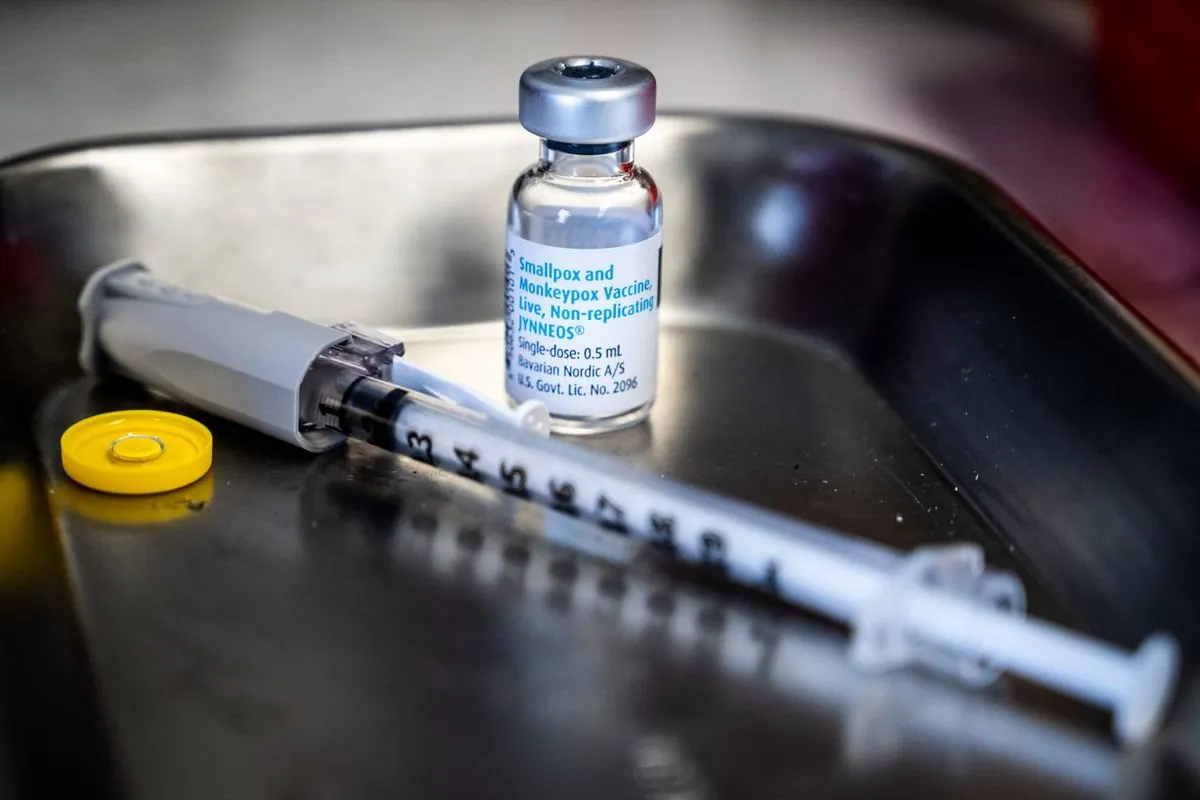
The World Health Organization (WHO) has taken a significant step in combating mpox by granting its inaugural authorization for an adult vaccine. This development, announced on September 13, 2024, marks a crucial advancement in the global effort to control the disease, particularly in Africa and other affected regions.
The pre-qualification of the vaccine, produced by Bavarian Nordic A/S, enables major donors such as GAVI the Vaccine Alliance and UNICEF to procure it. However, the availability remains constrained due to the presence of only one manufacturer.
Tedros Adhanom Ghebreyesus, WHO Director-General, emphasized the importance of this milestone, stating:
"This first pre-qualification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future."
He further stressed the urgent need to scale up procurement, donations, and distribution to ensure the vaccine reaches areas of greatest need, alongside other response measures.
The WHO authorization specifies that the vaccine is approved for individuals aged 18 and above, administered in a two-dose regimen. While not currently licensed for those under 18, the approval allows for its use in younger populations during outbreak situations where the benefits outweigh potential risks.
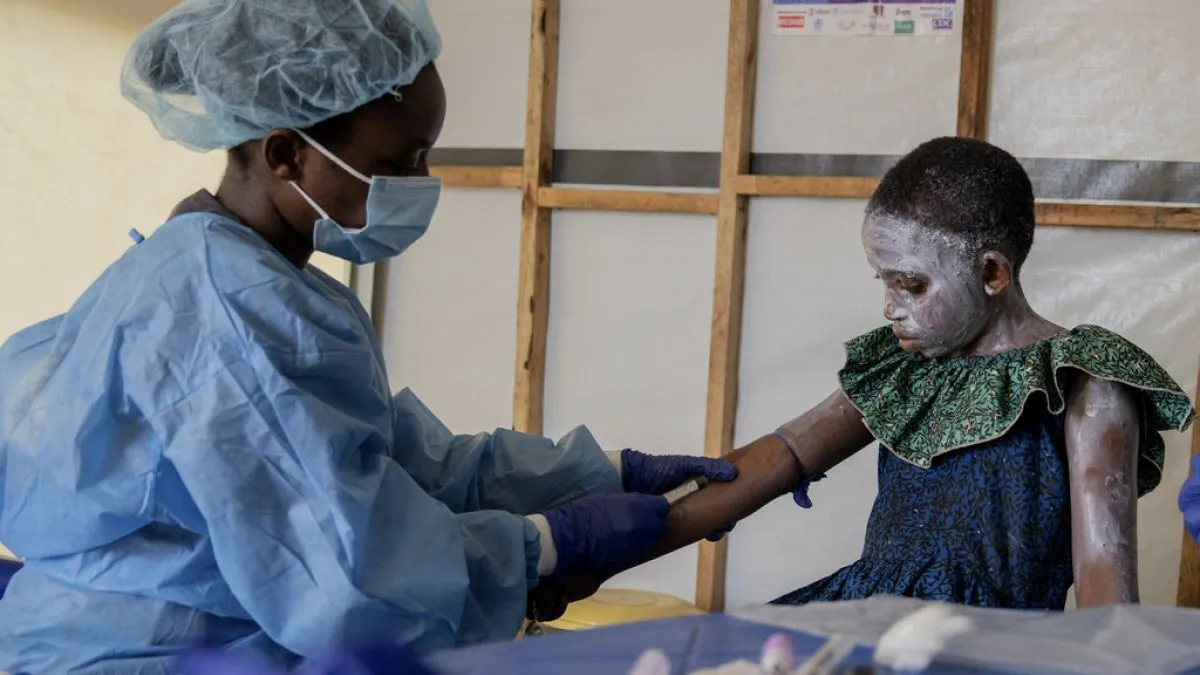
This provision is particularly relevant given the recent reports from the Africa Center for Disease Control and Prevention. In August 2024, officials noted that nearly 70% of mpox cases in Congo, the most severely affected country, were in children under 15. Alarmingly, this age group also accounted for 85% of mpox-related deaths in the country.
The situation in Africa remains critical. On September 8, 2024, the Africa CDC reported 107 new deaths and 3,160 new cases in the preceding week. This update came shortly after the launch of a continental response plan by the Africa CDC and WHO.
Mpox, first discovered in 1958 in a colony of research monkeys, belongs to the same virus family as smallpox but typically causes milder symptoms. These can include fever, chills, and body aches. In more severe cases, patients may develop lesions on the face, hands, chest, and genitals. The disease has a mortality rate of 1-10%, with higher rates observed in young children.
The 2022 mpox outbreak marked the first time the disease spread widely outside Africa, where it is endemic in certain regions, particularly rainforests. This global spread highlighted the need for improved prevention and control measures, including vaccination.
The smallpox vaccine, which is about 85% effective in preventing mpox, has been used in some cases. However, the development and authorization of a specific mpox vaccine represent a significant advancement in disease control efforts.
As environmental factors like deforestation potentially increase human-animal contact and mpox transmission, the need for effective vaccines becomes even more critical. The WHO's authorization of this vaccine is a pivotal step in addressing the ongoing outbreaks and preparing for future challenges in the fight against mpox.