FDA Denies MDMA for PTSD: Psychedelic Pioneer Vows to Continue Fight
The FDA rejected MDMA for PTSD treatment, dealing a blow to Rick Doblin's 30-year effort. Despite setbacks, Doblin remains committed to pursuing MDMA therapy globally and exploring new applications.

In a significant setback for psychedelic medicine, the U.S. Food and Drug Administration (FDA) has denied approval for MDMA as a treatment for post-traumatic stress disorder (PTSD). This decision, made in August 2024, marks a critical moment in the decades-long effort led by Rick Doblin to legitimize the therapeutic use of the substance also known as ecstasy.
Doblin, founder of the Multidisciplinary Association for Psychedelic Studies (MAPS), has been at the forefront of MDMA research since the 1980s. MAPS, established in 1986, has raised over $140 million to support research into psychedelic therapies. The organization's journey reflects the complex history of MDMA, which was first synthesized in 1912 by Merck and later gained popularity as a party drug in the 1980s before being classified as a Schedule I substance by the DEA in 1985.
Despite the FDA's rejection, which requires a new clinical study potentially costing millions of dollars and taking several years, Doblin remains undeterred. He criticized the agency for failing to recognize MDMA's potential to alleviate suffering and vowed to continue his efforts globally.
The FDA's decision highlights the challenges faced in psychedelic research, particularly regarding clinical trial design. One major obstacle was maintaining the "blinding" of participants, as MDMA's consciousness-altering effects made it difficult for subjects and therapists to remain unaware of whether they received the drug or a placebo. This issue raised concerns about potential bias in the study outcomes.
Doblin defended the integrity of the trials, pointing out that 47.6% of placebo group participants in one study no longer qualified for a PTSD diagnosis by the end of the trial, compared to 71.2% in the MDMA group. He argued that this demonstrated the therapists' commitment to helping all patients, regardless of their treatment assignment.
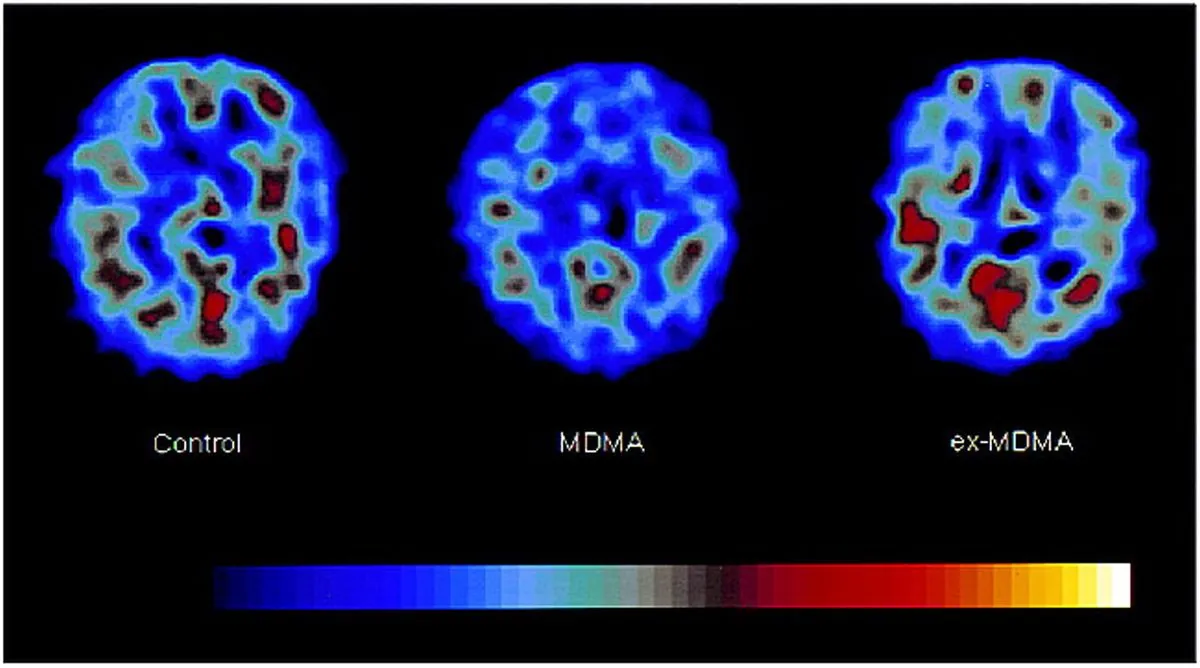
The research into MDMA-assisted therapy has shown promise, with the FDA granting it "breakthrough therapy" designation for PTSD treatment in 2017. MDMA is known to increase the release of serotonin, dopamine, and norepinephrine in the brain, typically producing effects lasting 3-6 hours. These neurochemical changes are believed to contribute to increased feelings of empathy and emotional openness, which may be beneficial in a therapeutic context.
"It's not about the drug, it's about the therapeutic context that the drug makes more effective."
As MAPS and its for-profit arm, Lykos Therapeutics, navigate this setback, Doblin is shifting focus to international initiatives. Projects are underway in Somaliland, Denmark (working with Syrian refugees), and Bosnia (assisting survivors of the Srebrenica massacre). Additionally, MAPS is supporting efforts to introduce MDMA therapy in Europe, bolstered by a Dutch state commission's recommendation for government approval.
While the road to FDA approval has hit a significant roadblock, the global landscape for MDMA research and therapy continues to evolve. Several countries, including the Netherlands and Canada, have considered or implemented MDMA therapy programs, reflecting a growing interest in the potential of psychedelic-assisted treatments for mental health disorders.
As the field of psychedelic medicine advances, researchers and advocates like Doblin continue to navigate the complex interplay of scientific rigor, regulatory requirements, and the potential to alleviate suffering for millions affected by PTSD and other mental health conditions.


































